Valence Electrons and Ionic Compounds
Emily Wilson
8 min read
Listen to this study note
Study Guide Overview
This study guide covers chemical bonding, focusing on valence electrons, charges of ions, electronegativity, and types of elements. It explains ionic and covalent bonds (both polar and nonpolar) with examples. It also discusses partial charges and provides practice questions and exam tips.
#AP Chemistry: Chemical Bonding - The Ultimate Study Guide 🚀
Hey there, future AP Chem master! Let's dive into the world of chemical bonding. This guide is designed to be your go-to resource, especially the night before the exam. We'll break down everything you need to know, make connections between topics, and get you feeling confident. Let's get started!
#⚛️ Foundational Knowledge of Bonding
#
Valence Electrons
- Valence electrons are the outermost electrons in an atom. They're the key players in chemical bonding.
- They reside in the s and p orbitals of the outermost shell.
- A jump in ionization energies can reveal the number of valence electrons.
- Elements in the same group (column) have the same number of valence electrons. This is a
crucial concept for predicting bonding behavior
.
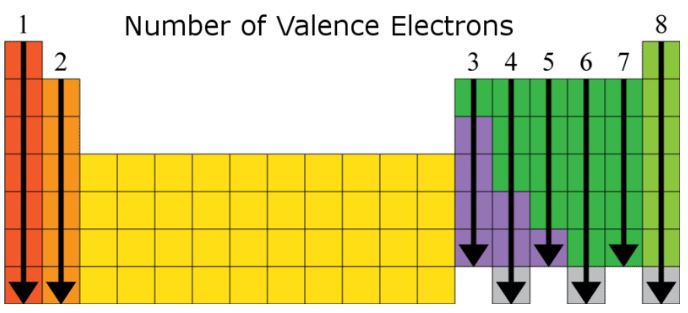
*Image Courtesy of ck12*
* The image above shows the number of valence electrons for main group elements. Transition metals are less predictable.
* **Oxygen (O)** has 6 valence electrons, and **Carbon (C)** has 4. Use the periodic table to quickly determine these numbers.
* Elements in the same group tend to bond similarly because of their similar valence electron configurations. For example:
* Group 1 elements bond with chlorine: LiCl, NaCl, KCl, RbCl
* Group 2 elements bond with oxygen: MgO, CaO, SrO, BaO
#
Charges of Ions
-
Ions are charged atoms or molecules that have gained or lost electrons. Atoms form ions to achieve a stable electron configuration.
-
Remember: Stability is the name of the game in chemistry! ⚖️

Image Courtesy of Chemistry Land
- Main group elements have predictable charges. Transition metals have variable charges. Don't worry, AP won't test you too much on transition metal charges.
#Types of Elements
-
There are three types of elements: metals, nonmetals, and metalloids.

Image Courtesy of ck12
- Metals: Good conductors of heat and electricity 🔥⚡, shiny, malleable, and ductile.
- Nonmetals: Poor conductors, brittle.
- Metalloids: Have properties of both metals and nonmetals. There are only 7 of them (B, Si, Ge, As, Sb, Te, and Po).
#
Electronegativity
- Electronegativity is how strongly an atom attracts electrons in a bond.
This is one of the five key periodic trends you MUST know.
- Fluorine (F) is the most electronegative element (4.0). Use its position on the periodic table as a reference point.
- As you move towards fluorine on the periodic table, electronegativity increases.
- 👉 Quick refresh? Check out our "Periodic Trends" study guide.
#🔗 Types of Bonds
Elements bond to achieve the lowest energy state and maximum stability. There are two main types of bonds:
#Ionic Bonds
- Ionic bonds involve the transfer of electrons, usually from a metal to a nonmetal.
- The atom that loses electrons becomes a cation (positive charge, usually a metal).
- The atom that gains electrons becomes an anion (negative charge, usually a nonmetal).
- Ionic compounds have strong bonds, are soluble in water, and conduct electricity when dissolved or molten.
#Example: NaCl
-
Sodium (Na) loses an electron to become Na⁺, while chlorine (Cl) gains an electron to become Cl⁻.

-
The octet rule states that atoms are most stable when they have 8 valence electrons. Group 1 and 17 elements bond to achieve this.
-
When forming an ionic bond with halogens, group one elements lose an entire electron shell.
-
Ions achieve the electron configuration of the nearest noble gas.

#Covalent Bonds
- Covalent bonds involve the sharing of electrons, usually between two nonmetals.
- Covalent compounds have low melting points and weak conductivity.
- There are two types of covalent bonds: polar and nonpolar.
- Polar covalent bonds: Unequal sharing of electrons due to different electronegativities.
- Nonpolar covalent bonds: Equal sharing of electrons due to similar electronegativities.
- Covalent bonds are strong and stable and are responsible for many common substances like water (H₂O), methane (CH₄), and even DNA.
#Polar Covalent Example: HF
-
Fluorine (F) is more electronegative than hydrogen (H), so it attracts electrons more strongly.

Image Courtesy of Study
- The dash represents two shared electrons. The electrons are pulled more towards fluorine.
#Nonpolar Covalent Example: Cl₂
-
Two chlorine atoms share electrons equally since they have the same electronegativity.

Image courtesy of Wayne Breslyn
-
Nonpolar bonds can also form between different elements if their electronegativities are similar. For example, C-H and C-O bonds are often considered nonpolar.
- Electronegativity of hydrogen: 2.2
- Electronegativity of carbon: 2.55
- Electronegativity of oxygen: 3.44
-
#⚡ Charges and Partial Charges
-
Nonpolar covalent bonds: Equal sharing, neutral charge distribution.
-
Polar covalent bonds: Unequal sharing, partial charges (δ+ and δ-).

Image Courtesy of Chem LibreTexts
- The Greek letter delta (δ) indicates a partial charge.
-
Ionic bonds: Complete transfer of electrons, full charges (+ and -).
-
Metals (low electronegativity) donate electrons and become cations.
-
Nonmetals (high electronegativity) accept electrons and become anions.
-
Example: Na (0.93) and Cl (3.16) have a large electronegativity difference, leading to full charges.
-
LEO says GER: Loss of Electrons is Oxidation, Gain of Electrons is Reduction.
#🎯 Final Exam Focus
- High-Priority Topics:
- Valence electrons and their role in bonding
- Electronegativity and its impact on bond polarity
- Distinguishing between ionic and covalent bonds
- Understanding partial and full charges
- Common Question Types:
- Predicting bond types based on electronegativity differences
- Determining the number of valence electrons
- Identifying the charges of ions
- Explaining the properties of ionic and covalent compounds
- Last-Minute Tips
- Review the periodic trends, especially electronegativity.
- Practice drawing Lewis structures to visualize electron sharing.
- Remember that the goal of bonding is to achieve stability (full octet).
- Don't forget to manage your time effectively on the exam. Start with questions you know well.
#🧪 Practice Questions
Practice Question
Multiple Choice Questions
-
Which of the following elements has the highest electronegativity? (A) Sodium (Na) (B) Chlorine (Cl) (C) Potassium (K) (D) Calcium (Ca)
-
What type of bond is formed between two oxygen atoms (O₂)? (A) Ionic (B) Polar covalent (C) Nonpolar covalent (D) Metallic
-
Which of the following compounds is most likely to exhibit ionic bonding? (A) CO₂ (B) H₂O (C) NaCl (D) CH₄
Free Response Question
Consider the molecules HF and F₂.
(a) Draw the Lewis structures for both molecules.
(b) Explain why HF is considered a polar molecule, while F₂ is nonpolar, in terms of electronegativity.
(c) Predict which molecule, HF or F₂, would have a higher boiling point and justify your answer.
Scoring Breakdown
(a) (2 points) - 1 point for correctly drawing the Lewis structure of HF (H-F with 6 lone pairs on F) - 1 point for correctly drawing the Lewis structure of F₂ (F-F with 3 lone pairs on each F)
(b) (2 points) - 1 point for stating that F is more electronegative than H, causing unequal sharing of electrons in HF. - 1 point for stating that F₂ has equal electronegativity, causing equal sharing of electrons.
(c) (2 points) - 1 point for correctly predicting that HF has a higher boiling point. - 1 point for justifying the answer by mentioning the presence of stronger dipole-dipole forces and hydrogen bonding in HF, which is absent in F₂.
Question Combining Multiple Units
- Given the compound magnesium chloride (MgCl₂), describe the type of bonding present, explain how the ions are formed, and predict the compound's solubility in water. Connect your explanation to the concepts of electronegativity and lattice energy.
Continue your learning journey

How are we doing?
Give us your feedback and let us know how we can improve





