Endothermic and Exothermic Processes
Caleb Thomas
8 min read
Listen to this study note
Study Guide Overview
This study guide covers thermodynamics focusing on energy, enthalpy, and heat flow. It reviews forms of energy (kinetic, potential, electrostatic), the law of conservation of energy, and how to study energy changes using the concepts of system, surroundings, and state functions. It explains endothermic and exothermic processes and relates them to enthalpy change (ΔH). Finally, it provides practice questions covering these concepts.
#Thermodynamics: Energy, Enthalpy, and the Flow of Heat
Welcome to your final review of thermodynamics! Let's make sure you're feeling confident and ready to tackle any energy-related question the AP exam throws your way. This guide is designed to be your go-to resource, especially the night before the big day. Let's dive in!
#
Forms of Energy
Remember from Unit 4, energy is the capacity to do work or transfer heat. It's all about movement and interactions, and it's the foundation of everything we'll discuss. Let's break down the key types:
# Kinetic Energy
- Definition: Energy of motion. Think of it as the energy of moving particles, like those in a gas. 💨
- Key Concept: Temperature is directly related to the average kinetic energy of particles. Higher temperature = faster particles.
- Formula: KE = ½mv², where:
-
m = mass (kg)
-
v = velocity (m/s)
-
KE is in Joules (J)
-
Don't stress about memorizing formulas! This is on your reference sheet. Focus on understanding how mass and velocity affect kinetic energy.
# Potential Energy
- Definition: Stored energy due to position or composition. In chemistry, we're mostly concerned with the energy stored in chemical bonds. ⚛️
- Key Concept: Lower potential energy = more stable compound. Systems always tend towards lower energy states.
#Electrostatic Energy
-
Definition: Potential energy due to the interaction of charged particles. This is all about Coulomb's Law in action!
-
Formula: PE = Q1Q2/d, where:
- Q1 and Q2 are the charges of the particles
- d is the distance between the charges
-
Key Concept: Opposite charges attract; like charges repel. Just like magnets! 🧲

Image Courtesy of NRG
# The Law of Conservation of Energy
-
Key Idea: Energy cannot be created or destroyed, only transferred or converted. This is also known as the First Law of Thermodynamics. 💡
-
Application: In any system, the total amount of energy remains constant. Think of a ball rolling down a hill: potential energy becomes kinetic energy, but the total energy stays the same.
This law is HUGE! It applies to all forms of energy and is the backbone of thermodynamics. Remember this for both MCQs and FRQs.
#
Studying Energy Changes
To understand energy changes, we need to define our terms:
# The System
- Definition: The specific part of the universe we're interested in (e.g., the reactants in a beaker).
- Types of Systems:
-
Open System: Exchanges both matter and energy with surroundings (e.g., a pot of boiling water). ⚖️
-
Closed System: Exchanges energy but not matter with surroundings (e.g., a sealed container).
-
Isolated System: Exchanges neither matter nor energy with surroundings (e.g., a calorimeter). 🌡️
-
Think of it like this: * Open: Everything flows (matter and energy). * Closed: Energy flows, but matter is stuck inside. * Isolated: Nothing flows in or out.
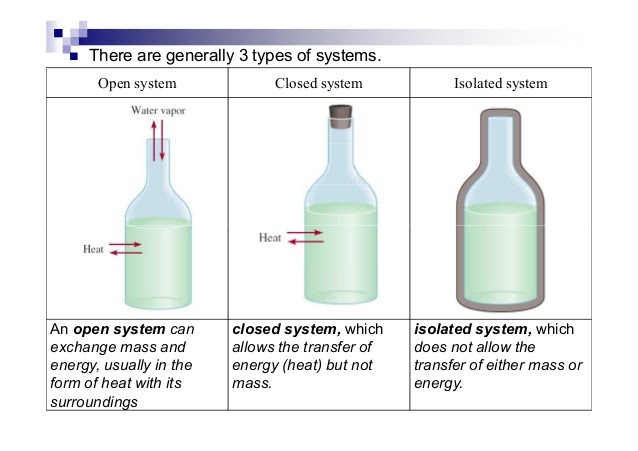
<center>Image Courtesy of Socratic</center>
# The Surroundings
- Definition: Everything else in the universe outside of the system (e.g., the beaker, the air, etc.).
- Key Concept: The system and surroundings always interact; energy is transferred between them.
# State Functions
-
Definition: Properties that depend only on the current state of the system, not how it got there. Examples include: energy, enthalpy, pressure, volume, and temperature.
-
Key Concept: Focus on initial and final states ONLY. The path doesn't matter. 🛣️
-
Non-State Functions: Heat and work are NOT state functions because they depend on the path taken.
Think of state functions like a road trip. The distance between your start and end points doesn't change, no matter which route you take. But the amount of gas you use (work) does depend on the route.
#
Endothermic vs Exothermic Processes
These terms describe how heat flows in a reaction. Let's get them straight:
#Enthalpy (H)
- Definition: The total heat content of a system. It's a state function. 🌡️
- ΔH (Delta H): The change in enthalpy. It's what we use to measure heat absorbed or released in a process.
# Endothermic Processes 🥵
-
Definition: Heat is absorbed by the system from the surroundings. Think of it as energy going into the system.
-
ΔH Value: +ΔH (positive). The system gains energy.
-
Key Concept: Heat flows into the system. The system feels cold to the touch.
ENDOthermic: ENergy DOes in.
# Exothermic Processes 🥶
-
Definition: Heat is released by the system to the surroundings. Think of it as energy going out of the system.
-
ΔH Value: -ΔH (negative). The system loses energy.
-
Key Concept: Heat flows out of the system. The system feels hot to the touch.
EXOthermic: EXergy Out.
Remember to think from the system's point of view. If the system gains energy (+ΔH), it's endothermic. If it loses energy (-ΔH), it's exothermic.
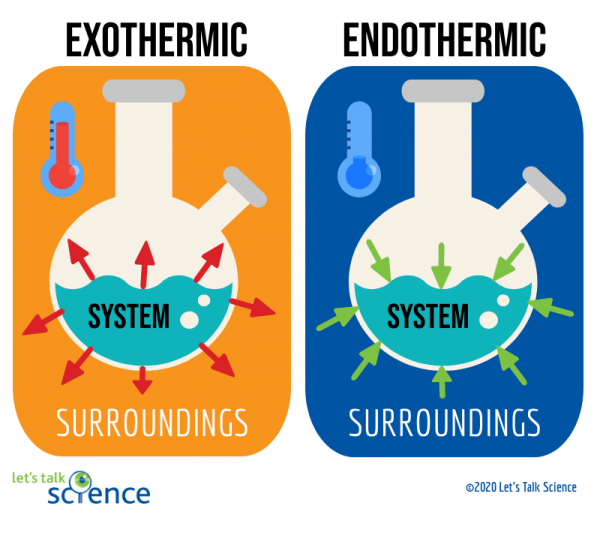
<center>Image Courtesy of Let's Talk Science</center>
#Hot and Cold Packs: Real-World Chemistry
-
Hot Packs: Use exothermic reactions to release heat. Example: Sodium acetate crystallizing when mixed with water. 🔥
-
Cold Packs: Use endothermic reactions to absorb heat. Example: Ammonium nitrate dissolving in water. ❄️
Understand the direction of heat flow in both endothermic and exothermic processes. This is a common MCQ and FRQ topic.
#Final Exam Focus
-
High-Priority Topics: Law of Conservation of Energy, system vs. surroundings, state functions, endothermic vs. exothermic processes, and enthalpy changes.
-
Common Question Types: MCQs that test definitions and concepts, FRQs that require calculations and explanations of energy flow.
-
Time Management: Quickly identify the system and surroundings, and use the correct sign (+ or -) for ΔH.
-
Common Pitfalls: Confusing endothermic and exothermic processes, and not focusing on the system's perspective.
Practice, practice, practice! Work through as many problems as you can. Make sure you are comfortable with the sign conventions for ΔH.
#Practice Questions
Practice Question
#Multiple Choice Questions
-
Which of the following statements is true regarding an endothermic reaction? (A) The enthalpy of the system decreases, and the surroundings become warmer. (B) The enthalpy of the system increases, and the surroundings become cooler. (C) The enthalpy of the system decreases, and the surroundings become cooler. (D) The enthalpy of the system increases, and the surroundings become warmer.
-
A 50.0 g piece of metal at 85.0°C is placed in 100.0 g of water at 22.0°C. The final temperature of the water and metal is 25.6°C. Assuming no heat is lost to the surroundings, which of the following can be determined from this experiment? (A) The specific heat capacity of the metal. (B) The enthalpy change of the water. (C) The kinetic energy of the metal. (D) The potential energy of the water.
-
Which of the following is NOT a state function? (A) Enthalpy (B) Temperature (C) Volume (D) Work
#Free Response Question
Consider the following reaction:
(a) Is this reaction exothermic or endothermic? Explain your reasoning.
(b) Draw a potential energy diagram for this reaction, labeling reactants, products, and the activation energy.
(c) If 100.0 g of reacts with excess , how much heat is released or absorbed? Show all your work.
(d) If the reaction is carried out in an open system, what would happen to the temperature of the surroundings? Explain.
#Scoring Breakdown
(a) (1 point)
- The reaction is exothermic because ΔH is negative.
(b) (2 points)
- Correctly labeled axes (Potential Energy vs. Reaction Progress) (1 point)
- Correctly labeled reactants, products, and activation energy. The products should be lower than the reactants (1 point)
(c) (3 points)
- Convert grams of to moles: 100.0 g / 28.02 g/mol = 3.57 mol (1 point)
- Use stoichiometry to find the heat released: 3.57 mol * (-92 kJ/mol) = -328.44 kJ (1 point)
- Correct answer with the correct sign and units (1 point)
(d) (1 point)
- The temperature of the surroundings would increase because the reaction is exothermic and releases heat into the surroundings.
You've got this! Remember to stay calm, read each question carefully, and trust in your preparation. You've covered a lot, and you're ready to show off what you know. Good luck! 🍀
Continue your learning journey

How are we doing?
Give us your feedback and let us know how we can improve





