Introduction to Equilibrium
Emily Wilson
7 min read
Listen to this study note
Study Guide Overview
This study guide covers chemical equilibrium, including reversible reactions (represented with ⇌), the concept of dynamic equilibrium where the rate of forward and reverse reactions are equal, and the requirement of a closed system. It explains the equilibrium constants Kc (concentration-based) and Kp (pressure-based), their relationship, and how to calculate them. The guide also emphasizes that equilibrium is a dynamic process, not a static one, and provides practice questions covering these concepts.
#Chemical Equilibrium: The Ultimate Study Guide 🚀
Welcome to the world of chemical equilibrium! This guide will transform your understanding of reversible reactions, equilibrium constants, and everything you need to ace your AP Chemistry exam. Let's dive in!
#Introduction to Reversible Reactions
#What are Reversible Reactions? 🔄
Reversible reactions are reactions that can proceed in both the forward (reactants to products) and reverse (products to reactants) directions simultaneously. This is a key concept that builds upon your knowledge from Units 4 and 5. Think of it like a two-way street, where reactants and products are constantly interchanging. 🚦

- Examples of Reversible Processes:
- Evaporation and condensation of water: H₂O(l) ⇌ H₂O(g)
- Dissolution and precipitation of a salt: NH₄Cl(s) ⇌ NH₄⁺(aq) + Cl⁻(aq)
- Acid-base reactions: H₂CO₃ + HCO₃⁻ ⇌ H₂O + CO₂
- Redox reactions: Zn + Cu²⁺ ⇌ Zn²⁺ + Cu
#The Double Arrow ⇌
- The double arrow (⇌) is the symbol for a reversible reaction and indicates a system at equilibrium.
- Use the correct arrows in free-response questions: * Single arrow (→) for reactions in one direction. * Double arrow (⇌) for reversible reactions at equilibrium.
#Understanding Equilibrium
#What is Equilibrium? 🤔
Equilibrium is the state where the rate of the forward reaction equals the rate of the reverse reaction. It's a dynamic process, not a static one. Imagine a busy marketplace where goods are constantly being bought and sold, but the overall amount of goods remains the same. ⚖️
- Rate of Forward Reaction = Rate of Reverse Reaction
- As reactant concentrations decrease, the forward reaction rate slows down.
- As product concentrations increase, the reverse reaction rate speeds up.
#Equilibrium Graphically

- Equilibrium State: The point where the rates of the forward and reverse reactions are equal.
- Key Insight: At equilibrium, concentrations of reactants and products remain constant, but the reaction is still happening. Rates are equal, concentrations are constant.
#Closed System Requirement
Equilibrium can only occur in a closed system. This is a system that does not exchange matter or energy with its surroundings. Think of it as a sealed container where nothing can escape or enter. 📦

- Closed System: Fixed concentrations of reactants and products.
- Open System: Matter and/or energy can be exchanged, preventing equilibrium.
#Measuring Equilibrium: Kc and Kp
#Equilibrium Constant (Kc)
Kc is a value that measures how far a reaction proceeds toward products at equilibrium. It's the ratio of product concentrations to reactant concentrations at equilibrium. 🧪
- Rate Laws:
-
Forward reaction: A → B Rate = k₁[A]
-
Reverse reaction: B → A Rate = k₂[B]
-
At equilibrium: k₁[A] = k₂[B]
-

- Kc = [Products]/[Reactants] at equilibrium
- Kc is unitless and changes with temperature.
- Initial reactant and product concentrations do NOT change Kc.
#Equilibrium Constant (Kp)
Kp is similar to Kc but uses partial pressures instead of concentrations, specifically for gases. It's another way to measure equilibrium. 💨
- Kp is calculated using partial pressures of products and reactants at equilibrium.
- Both Kc and Kp represent the extent of a reaction at equilibrium.
#Relationship between Kp and Kc
The relationship between Kp and Kc is given by the formula:
where Δn is the change in the number of moles of gas (moles of gaseous products - moles of gaseous reactants). This formula is less common on the exam but good to know. 💡
#Common Misconception: Equilibrium is NOT a Stopped Reaction
- Equilibrium is not when a reaction stops. It's a dynamic process where reactants and products are constantly interconverting.
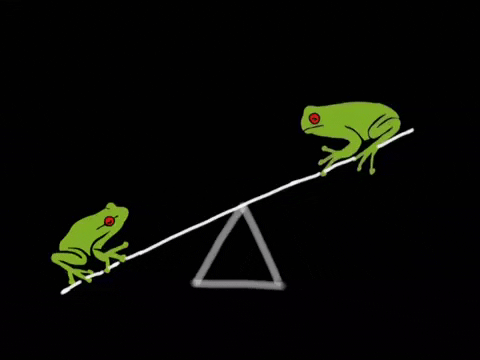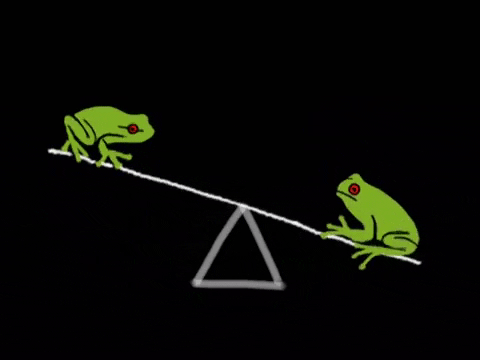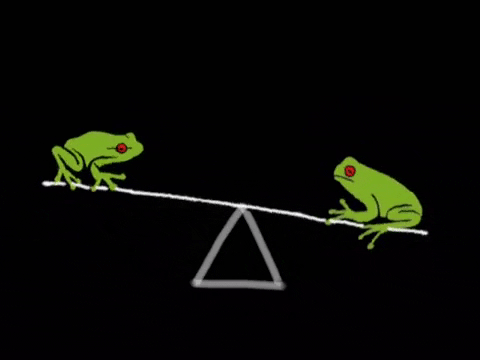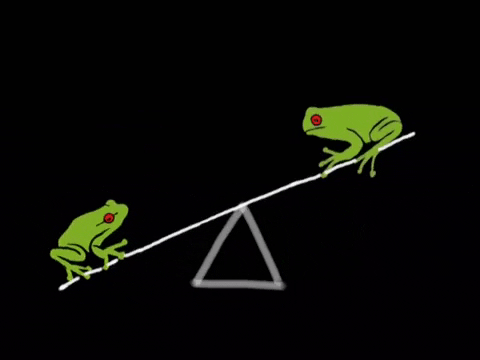
- Reactants and products are constantly changing, but concentrations remain constant.
- The system is active, but there are no observable changes.
#Final Exam Focus
-
- Key Topics: Reversible reactions, equilibrium definition, closed systems, Kc, and Kp.
- Common Question Types:
- Conceptual questions about equilibrium and dynamic processes.
- Calculations involving Kc and Kp (especially in later units).
- Interpreting graphs of reaction rates and concentrations.
- Last-Minute Tips:
- Understand the difference between equal rates and equal concentrations at equilibrium.
- Pay attention to the state symbols (g, l, s, aq) when writing equilibrium expressions.
- Practice interpreting graphs showing changes in concentrations over time.
#Practice Questions
Practice Question
#Multiple Choice Questions
-
Which of the following statements is true regarding a system at equilibrium? (A) The rates of the forward and reverse reactions are zero. (B) The rate of the forward reaction is equal to the rate of the reverse reaction. (C) The concentrations of reactants and products are equal. (D) The reaction has stopped.
-
For the reaction N₂(g) + 3H₂(g) ⇌ 2NH₃(g), what is the correct expression for the equilibrium constant, Kc? (A) Kc = [NH₃] / [N₂][H₂] (B) Kc = [N₂][H₂] / [NH₃] (C) Kc = [NH₃]² / [N₂][H₂]³ (D) Kc = [N₂][H₂]³ / [NH₃]²
-
A reaction is at equilibrium in a closed container. If more reactant is added, what will happen to the system? (A) The system will remain at equilibrium. (B) The rate of the forward reaction will temporarily increase. (C) The rate of the reverse reaction will temporarily increase. (D) The equilibrium constant will increase.
#Free Response Question
Consider the following reaction at equilibrium:
CO(g) + H₂O(g) ⇌ CO₂(g) + H₂(g)
At a certain temperature, the equilibrium concentrations are found to be:
[CO] = 0.200 M [H₂O] = 0.500 M [CO₂] = 0.300 M [H₂] = 0.900 M
(a) Write the expression for the equilibrium constant, Kc, for the reaction. (b) Calculate the value of the equilibrium constant, Kc. (c) If the volume of the container is doubled, what will happen to the value of Kc? Explain. (d) If the temperature of the system is increased, and the reaction is exothermic, what will happen to the value of Kc? Explain.
#Scoring Breakdown:
(a) 1 point for correct expression: Kc = [CO₂][H₂] / [CO][H₂O] (b) 1 point for correct substitution: Kc = (0.300)(0.900) / (0.200)(0.500) 1 point for correct calculation: Kc = 2.7 (c) 1 point for stating that Kc will not change. 1 point for correct explanation: Kc is independent of volume changes at constant temperature. (d) 1 point for stating that Kc will decrease. 1 point for correct explanation: In an exothermic reaction, increasing the temperature favors the reverse reaction, thus decreasing the value of Kc.
This study guide is designed to be your ultimate resource for mastering chemical equilibrium. Keep reviewing, stay confident, and you'll ace your AP Chemistry exam! Good luck! 🍀
Continue your learning journey

How are we doing?
Give us your feedback and let us know how we can improve





