Ocean Acidification
Jack Wilson
7 min read
Listen to this study note
Study Guide Overview
This study guide covers ocean acidification, including: the chemical process behind it (CO2 reacting with seawater), its impact on marine life (calcium carbonate availability, shell formation, fish physiology), the specific case of pteropods, and human impact through fossil fuels and deforestation. It also provides practice questions and exam tips.
#Ocean Acidification: A Deep Dive 🌊
Hey there, future AP Environmental Science rockstar! Let's break down ocean acidification, a crucial topic that often pops up on the exam. We'll make sure you're not just memorizing facts, but truly understanding the connections. Let's get started!
#What is Ocean Acidification?
Ocean acidification is the ongoing decrease in the pH of the Earth's oceans, caused by the uptake of carbon dioxide (CO2) from the atmosphere. Think of it like this: the ocean is like a giant sponge, soaking up our excess CO2. But this "soaking" has consequences. 🧪
#The Chemistry Behind It
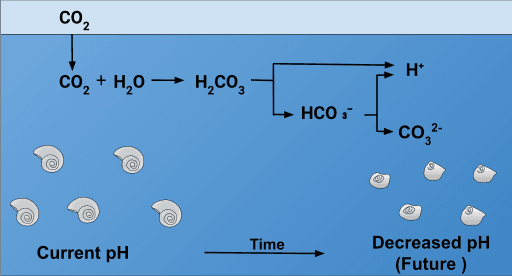
When CO2 dissolves in seawater, it reacts with water to form carbonic acid (H2CO3). This carbonic acid then releases hydrogen ions (H+), which increases the acidity (lowers the pH) of the ocean. Here's the reaction:
- Key Point: Increased atmospheric CO2 leads to increased ocean acidity.
#Human Impact
- Quick Fact: The burning of fossil fuels and deforestation are major contributors to increased atmospheric CO2.
Over the past 200 years, the ocean's pH has dropped by 0.1 units. While that might not sound like much, remember that the pH scale is logarithmic, meaning a 0.1 change represents about a 30% increase in acidity! 📉
- Memory Aid: Think of the pH scale like the Richter scale for earthquakes. Each whole number represents a tenfold change. A small change in pH means a BIG change in acidity.
#Impacts of Changing pH
So, what does this increased acidity mean for marine life? Let's dive in!
#Calcium Carbonate Crisis
- High-Value Topic: This is a HUGE area for AP questions, so pay close attention!
Increased acidity reduces the availability of calcium carbonate (CaCO3), a crucial building block for many marine organisms. Here's why it matters:
- Shell Formation: Organisms like corals, clams, oysters, and snails use calcium carbonate to build their shells and skeletons. Acidification makes it harder for them to extract the necessary calcium carbonate from the water. 🐚
- Protection & Buoyancy: These structures are vital for protection from predators and maintaining the organism's shape and buoyancy.
- Internal pH Regulation: Calcium carbonate is also used by many organisms to control their internal pH and calcium levels.
- Common Mistake: Don't confuse ocean acidification with ocean pollution. They are related but different issues. Acidification is about pH, pollution is about contaminants.
#Fish Physiology
Acidification can mess with fish in several ways:
- Sense of Smell: Many fish rely on their sense of smell to find food, mates, and avoid predators. Increased acidity can disrupt their ability to detect these crucial odors. 🐠
- Physiological Stress: Acidic conditions can cause physiological stress in fish, affecting their overall health and survival.
#Aquatic Plants
Interestingly, some organisms benefit from increased CO2:
- Seagrass and Algae: These plants tend to grow more in a CO2-rich environment. However, this can lead to imbalances.
- Hypoxic Environments: If plant growth explodes and herbivores can't keep up, it can lead to eutrophication and hypoxic (low oxygen) conditions.
#The Pteropod Problem
Let's talk about a specific example: pteropods. These tiny sea snails are a big deal because they are a major food source for many animals. 🌀
#Why Pteropods Matter
- Food Chain Base: Pteropods form the base of the food chain for many species, including whales and salmon.
- Shell Dissolution: Studies have shown that pteropod shells can dissolve in less than 50 days in waters with predicted acidity levels. This is a HUGE problem.
- Domino Effect: The collapse of pteropod populations would have a ripple effect throughout the marine ecosystem.
#Predicted Future
Scientists predict that ocean acidity could increase by over 100% in the next 100 years if CO2 production continues at its current rate. This would have devastating consequences for marine life and the human populations that depend on it. 😥

#Image Courtesy of Wikimedia

#Image courtesy of Wikimedia
#Image Courtesy of Wikimedia
#Final Exam Focus
Okay, you've made it! Here's what to focus on for the exam:
- The Chemistry: Understand the reaction of CO2 with seawater and how it leads to increased acidity.
- Calcium Carbonate: Know why calcium carbonate is important and how acidification affects its availability.
- Food Chain Impacts: Be ready to discuss how acidification impacts various marine organisms, especially those at the base of the food chain.
- Human Impacts: Connect ocean acidification to human activities like fossil fuel burning and deforestation.
#Exam Tips
- Exam Tip: When answering FRQs, be specific. Don't just say "it hurts marine life." Explain how it hurts marine life. Use scientific terms like "calcium carbonate" and "pH."
- Time Management: Don't spend too long on one question. If you get stuck, move on and come back later.
- Read Carefully: Pay close attention to the wording of the questions. They often contain clues to the answer.
- Don't Panic: You've got this! Take deep breaths and trust your knowledge.
#Practice Questions
Practice Question
#Multiple Choice Questions
-
Which of the following is the primary cause of ocean acidification? (a) Increased volcanic activity (b) Absorption of excess atmospheric CO2 (c) Runoff from agricultural fields (d) Thermal pollution from power plants
-
What is the main effect of ocean acidification on marine organisms that build shells? (a) Increased shell growth (b) Decreased shell growth and maintenance (c) Enhanced ability to regulate internal pH (d) Improved predator avoidance
-
Which of the following organisms is most vulnerable to the effects of ocean acidification? (a) Seagrass (b) Algae (c) Pteropods (d) Fish
#Free Response Question
Scenario: A coastal community is experiencing a decline in shellfish populations. Scientists have determined that ocean acidification is a contributing factor.
(a) Explain the chemical process that leads to ocean acidification. (3 points)
(b) Describe two specific ways that ocean acidification affects shellfish populations. (4 points)
(c) Discuss one potential consequence of the decline in shellfish populations on the marine food web. (2 points)
(d) Suggest one realistic strategy that could be implemented to mitigate the effects of ocean acidification in this coastal community. (2 points)
#FRQ Scoring Breakdown
(a)
- (1 point) CO2 from the atmosphere dissolves in seawater.
- (1 point) This forms carbonic acid (H2CO3).
- (1 point) Carbonic acid releases hydrogen ions (H+), which lowers the pH.
(b)
- (2 points) Reduced availability of calcium carbonate (CaCO3) for shell formation.
- (2 points) Difficulty in maintaining existing shells, leading to weaker or dissolving shells.
(c)
- (2 points) Decline in shellfish populations can lead to a decrease in food availability for predators, disrupting the food web.
(d)
- (2 points) One realistic strategy, such as reducing local carbon emissions, restoring coastal wetlands, or implementing carbon capture technologies.
Alright, you've got this! Go ace that exam!
Continue your learning journey

How are we doing?
Give us your feedback and let us know how we can improve





