Atomic Structure and Properties
Question 1
mock_examChemistryAPZuAI Powered
Which equation shows the enthalpy of formation, ∆Hf, of ethanol?
A
2C s+3H2 g+12O2 g→C2H5OH g
B
4C s+6H2 g+O2 g→2C2H5OH g
C
2C s+3H2 g+12O2 g→C2H5OH l
D
4C s+6H2 g+O2 g→2C2H5OH l
Question 2
2018ChemistryAPConcept Practice
Answer the following questions relating to Fe and its ions, and .
Question 3
2018ChemistryAPConcept Practice
The complete photoelectron spectrum of an element is represented above.
Question 4
2019ChemistryAPConcept Practice
The complete photoelectron spectrum of an element in its ground state is represented below.
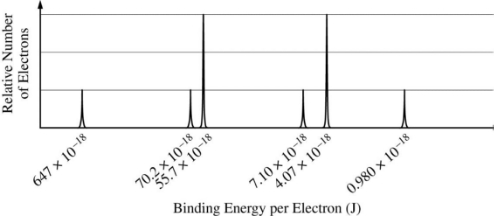
5.a. (2 points) Based on the spectrum,
5.a.i. (1 point) write the ground-state...

How are we doing?
Give us your feedback and let us know how we can improve