Solubility
Emily Wilson
7 min read
Listen to this study note
Study Guide Overview
This study guide covers solubility including definitions of key terms like solute, solvent, and homogeneous mixture. It explores the principle of "like dissolves like" and intermolecular forces (IMFs). It also examines saturation, saturation points, and interpreting solubility curves. Finally, it discusses factors affecting solubility such as polarity, pressure, temperature, and other factors like concentration and surface area.
#Solutions and Solubility: Your Night-Before-the-Exam Guide
Hey there, future AP Chem master! Let's dive into solutions and solubility. Remember, it's all about how substances mix and interact. You've got this!
#
What is Solubility?
#The Basics
-
Solubility is the ability of a substance (solute) to dissolve in another (solvent) to form a homogeneous mixture (a solution).
-
"Like dissolves like": Polar solutes dissolve in polar solvents, and non-polar solutes dissolve in non-polar solvents.
-
This is all about intermolecular forces (IMFs). Similar IMFs = higher solubility. 💡
Think of it like a dance party. Similar dancers (IMFs) are more likely to pair up and mingle (dissolve).
#Solubility Rules
-
Certain ions tend to form soluble compounds, while others tend to form insoluble ones. (See image below)
-
Don't try to memorize every single exception! You'll get the hang of it through practice. 💪
Focus on understanding the general trends rather than memorizing every single rule. You'll implicitly learn them through practice problems.
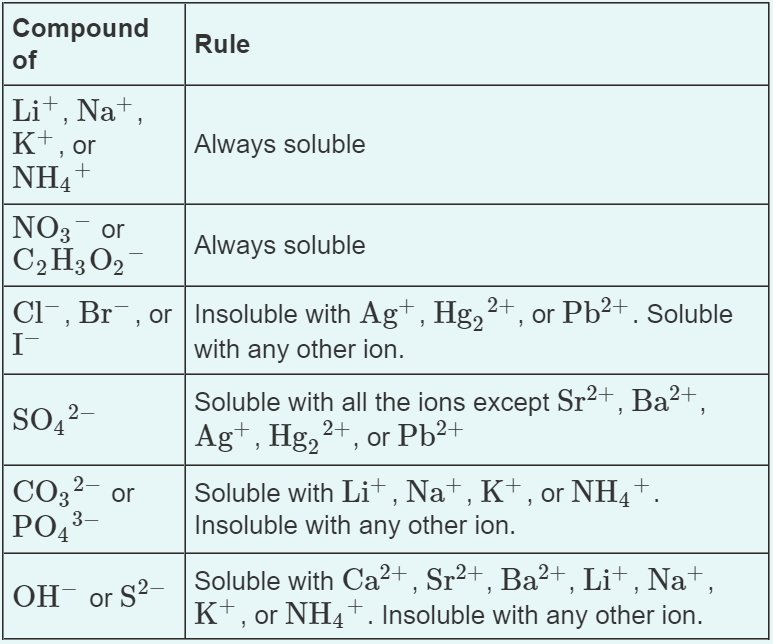
*Image Courtesy of Quizlet*
Common exceptions to solubility rules include ions like Ag+, Hg2 2+, and Pb2+.
#
Saturation of Solutions
#Saturation Point
- Every solution has a saturation point: the maximum amount of solute that can dissolve in a solvent at a given temperature.
- This point depends on temperature, the solvent, and the solute.
- Generally, higher temperatures allow more solute to dissolve (for most solids and liquids).
#Solubility Curves
-
These graphs show how solubility changes with temperature.
-
X-axis: Temperature (°C)
-
Y-axis: Mass of solute dissolved (usually in g/100 mL of solvent)

Image Courtesy of Dynamic Science
Practice reading these graphs! Pay attention to the axes and what they represent.
#Types of Solutions
-
Saturated Solution: Contains the maximum amount of solute at a given temperature. It's on the solubility curve. ⚖️
-
Undersaturated Solution: Contains less solute than the maximum. It's below the solubility curve. ⬇️
-
Supersaturated Solution: Contains more solute than it should at that temperature. It's above the solubility curve and often unstable. ⬆️
Think of it like filling a glass of water: undersaturated is not full, saturated is just full, and supersaturated is overflowing.

*GIF Courtesy of Gyfcat*
#Factors Affecting Solubility
#Polarity
- "Like dissolves like" is key! Polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes.
#Pressure
-
Only affects gases.
-
Higher pressure = higher gas solubility (think of a soda can). 🥤
-
Henry's Law: C = kP (not essential for the AP exam, but good to know!)
Pressure changes have negligible effects on the solubility of solids and liquids.
#Temperature
-
Solids/Liquids: Higher temperature = higher solubility.
-
Gases: Higher temperature = lower solubility. (We'll revisit this in Unit 7!).
Temperature has opposite effects on the solubility of gases compared to solids and liquids.
#Other Factors
- Concentration of the Solvent: More solvent usually means more solute can dissolve.
- Presence of Other Substances: Can affect solubility by altering the solvent's chemical nature or competing for binding sites.
- Surface Area: Smaller solute particles (larger surface area) generally increase solubility.
#
Final Exam Focus
#Key Topics
- Intermolecular forces (IMFs) and their role in solubility.
- Solubility rules (especially common exceptions).
- Reading and interpreting solubility curves.
- Understanding the differences between saturated, unsaturated, and supersaturated solutions.
- Factors affecting solubility: temperature, pressure, polarity, surface area.
#Common Question Types
- Multiple Choice: Identifying soluble/insoluble compounds, interpreting solubility curves, predicting the effect of temperature/pressure on solubility.
- Free Response: Explaining solubility trends, designing experiments to test solubility, analyzing experimental data related to saturation.
#Last-Minute Tips
-
Time Management: Don't spend too long on one question. Move on and come back if needed.
-
Common Pitfalls: Double-check units, pay attention to phases (solid, liquid, gas), and don't forget about IMFs!
-
Strategies: Read the question carefully, identify key concepts, and show all your work for partial credit.
Focus on understanding the concepts rather than rote memorization. Apply your knowledge to different scenarios.
#Practice Questions
Practice Question
#Multiple Choice Questions
-
Which of the following compounds is most likely to be soluble in water? (A) (B) (C) (D)
-
According to the solubility curve, how many grams of can dissolve in 100 g of water at 50°C? (A) 50 g (B) 80 g (C) 100 g (D) 120 g
-
A gas is dissolved in water. Which of the following changes will increase the solubility of the gas? (A) Increasing the temperature and decreasing the pressure (B) Increasing the temperature and increasing the pressure (C) Decreasing the temperature and decreasing the pressure (D) Decreasing the temperature and increasing the pressure
#Free Response Question
A student is given a solid sample of an unknown salt. The student performs experiments to determine the solubility of the salt in water at different temperatures. The data is shown below:
| Temperature (°C) | Solubility (g/100 g H2O) |
|---|---|
| 20 | 30 |
| 40 | 45 |
| 60 | 60 |
| 80 | 75 |
(a) Plot the data on a graph. Label the axes and include units.
(b) Based on the data, is the dissolution of this salt endothermic or exothermic? Explain your reasoning.
(c) If 50 g of the salt is added to 100 g of water at 30°C, would the solution be saturated, unsaturated, or supersaturated? Explain.
(d) If the solution from part (c) is heated to 70°C, how much additional salt can be dissolved in the solution?
#Scoring Breakdown
(a) (3 points) - 1 point for correctly labeled x-axis with temperature (°C) - 1 point for correctly labeled y-axis with solubility (g/100 g H2O) - 1 point for plotting the data correctly
(b) (2 points) - 1 point for stating the dissolution is endothermic - 1 point for explaining that solubility increases with temperature
(c) (2 points) - 1 point for stating the solution is unsaturated - 1 point for explaining that at 30°C, the solubility is between 30 and 45 g/100g H2O
(d) (2 points) - 1 point for calculating the solubility at 70°C (approximately 67.5 g/100g H2O) - 1 point for determining that an additional 17.5 g of salt can dissolve in the solution
Alright, you've got this! Remember to breathe, stay calm, and trust your knowledge. You're ready to rock this AP Chemistry exam! 🎉
Continue your learning journey

How are we doing?
Give us your feedback and let us know how we can improve





