Thermodynamic and Kinetic Control
Ethan Taylor
7 min read
Listen to this study note
Study Guide Overview
This study guide covers kinetic control, explaining why spontaneous reactions (negative ΔG) can be slow. It reviews thermodynamics vs. kinetics, reaction rates, rate laws, and activation energy (Ea). The guide emphasizes the importance of Ea in determining reaction speed and uses the diamond-to-graphite conversion as a key example. Finally, it discusses how catalysts lower Ea, increasing reaction rates, and provides practice questions covering these concepts.
#Kinetic Control: Why Spontaneous Reactions Can Be Slow 🐌
Hey AP Chem student! Let's dive into why some reactions, even if they're supposed to happen, take forever. We're talking about kinetic control, a concept that's super important for understanding how reactions actually behave. Get ready to make some connections between thermodynamics and kinetics!
#
Thermodynamics vs. Kinetics: A Quick Recap
Before we jump in, let's quickly review the difference between thermodynamics and kinetics:
- Thermodynamics: Tells us if a reaction can happen (spontaneous/non-spontaneous). Think Gibbs Free Energy (ΔG).
- Kinetics: Tells us how fast a reaction happens. Think reaction rates and activation energy.
It's crucial to remember that a negative ΔG (spontaneous reaction) doesn't guarantee a fast reaction! This is where kinetic control comes in.
#Brief Review of Kinetics
Okay, let's make sure we're all on the same page with kinetics. If you're feeling good, you can skim this, but a quick refresh never hurts!
-
Reaction Rate (R): How quickly reactants turn into products. It's measured as the change in concentration over time:
- or
-
Rate Laws: These equations relate reactant concentrations to the reaction rate:
-
(where k is the rate constant, and n and m are reaction orders)
-
Example: If rate = k[A], the reaction rate depends directly on the concentration of A.
-
-
Activation Energy (Ea): The minimum energy needed for a reaction to occur. Think of it as the hill reactants need to climb to become products. ⛰️
- Higher Ea = Slower reaction

#A Shortcoming of Gibbs Free Energy
Here's the kicker: Gibbs Free Energy (ΔG) only tells us if a reaction is possible, not how fast it will go. A negative ΔG means the reaction is spontaneous, but it doesn't guarantee it will happen quickly.
Don't assume that a spontaneous reaction (negative ΔG) will occur at an observable rate. Many spontaneous reactions are very slow!
#The Diamond-Graphite Example
Let's take a classic example: the conversion of diamonds to graphite:
- ΔG° = -3 kJ (spontaneous!)
- But, does your diamond ring suddenly turn into graphite? Nope!
- This reaction is incredibly slow (thousands of years!). It's under kinetic control.
Think of it like this: A ball rolling downhill (negative ΔG) is spontaneous, but if there's a huge mountain in the way (high Ea), it'll take forever to get there. 🏔️
#Reasons For Kinetic Control
So, why are some spontaneous reactions so slow? The main reason is a high activation energy (Ea).
- Even if a reaction is thermodynamically favorable, it won't proceed at a measurable rate if there's a large energy barrier to overcome.
#Catalysts: The Speed Boosters 🚀
Luckily, there's a way to speed up these reactions: catalysts! Catalysts work by:
- Providing an alternative reaction pathway with a lower activation energy.
- They don't change ΔG, they just make the reaction go faster.
#The Hydrogen Peroxide Example
-
Decomposition of hydrogen peroxide is normally very slow.
-
But with an iodide catalyst, it becomes the famous "Elephant's Toothpaste" reaction!

Remember, catalysts don't change the thermodynamics (ΔG) of a reaction, only the kinetics (rate).
#Getting Out of Kinetic Control
Catalysts help reactions escape kinetic control by lowering the activation energy. If we had a catalyst for the diamond-to-graphite reaction, it would happen much faster!
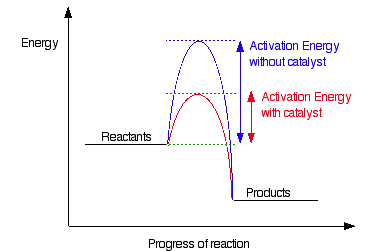
*Caption: Catalysts lower the activation energy, speeding up reactions.*
#Final Exam Focus
Okay, you've made it! Here's what to focus on for the exam:
- Distinguish between thermodynamic favorability (ΔG) and reaction rate (kinetics). Don't mix them up!
- Understand activation energy (Ea) and how it affects reaction rate. Higher Ea = slower reaction.
- Know how catalysts work. They lower Ea, speeding up reactions without changing ΔG.
- Be able to explain kinetic control using examples like the diamond-graphite conversion.
#Last-Minute Tips
- Time Management: Don't spend too long on one question. If you're stuck, move on and come back later.
- Common Pitfalls: Watch out for questions that try to trick you by mixing up thermodynamics and kinetics.
- FRQ Strategy: Clearly explain your reasoning. Show all your work for calculations.
#Practice Questions
Practice Question
Multiple Choice Questions
-
Which of the following statements is TRUE regarding a reaction with a negative ΔG°? (A) The reaction will proceed quickly. (B) The reaction is spontaneous. (C) The reaction requires a catalyst to occur. (D) The reaction is non-spontaneous.
-
A catalyst increases the rate of a reaction by: (A) Increasing the activation energy. (B) Decreasing the activation energy. (C) Increasing the ΔG° of the reaction. (D) Decreasing the ΔG° of the reaction.
-
A reaction is found to have a very high activation energy. Which of the following is the most likely consequence? (A) The reaction will be very fast. (B) The reaction will be very slow. (C) The reaction will be exothermic. (D) The reaction will be endothermic.
Free Response Question
The following reaction is spontaneous at room temperature:
However, the reaction proceeds very slowly under normal conditions. When a small amount of iodide ions () is added to the solution, the reaction proceeds rapidly.
(a) Explain why the reaction is slow under normal conditions, even though it is spontaneous.
(b) Explain how the iodide ions act as a catalyst in this reaction.
(c) Draw a potential energy diagram for the reaction, both with and without the catalyst. Label the axes, reactants, products, activation energy (), and the change in enthalpy (ΔH).
Scoring Breakdown
(a) (2 points)
- 1 point for stating that the reaction is slow due to a high activation energy.
- 1 point for relating the high activation energy to kinetic control.
(b) (2 points)
- 1 point for stating that the iodide ions provide an alternative reaction pathway.
- 1 point for mentioning that the new pathway has a lower activation energy.
(c) (4 points)
- 1 point for correctly labeled axes (Potential Energy vs. Reaction Progress).
- 1 point for correctly showing the reactants and products.
- 1 point for showing two curves: one with a higher Ea (uncatalyzed) and one with a lower Ea (catalyzed).
- 1 point for correctly labeling Ea and ΔH (or ΔE) on the diagram.
Good luck, you've got this! 🚀
Continue your learning journey

How are we doing?
Give us your feedback and let us know how we can improve





