Kinetic Molecular Theory
Sophie Anderson
8 min read
Study Guide Overview
This study guide covers the Kinetic Molecular Theory (KMT), including its five assumptions, and Maxwell-Boltzmann distributions. It explains the relationship between temperature, kinetic energy, and molecular speed. The guide also provides practice applying these concepts to AP exam-style questions, including a breakdown of a 2019 free-response question. Ideal gas behavior and PLIGHT conditions are also discussed.
#Kinetic Molecular Theory & Maxwell-Boltzmann Distributions 🚀
Hey there, future AP Chem master! Let's dive into the world of ideal gases and the amazing Kinetic Molecular Theory (KMT). This is your go-to guide for acing those tricky gas questions, especially the night before the exam. Remember, you've got this!
This topic is crucial for understanding gas behavior and often appears in both multiple-choice and free-response questions. It's a foundational concept, so make sure you nail it!
# Kinetic Molecular Theory (KMT)
Ideal gases are theoretical gases that perfectly follow the ideal gas law. The KMT helps us understand their behavior. Think of it as the rulebook for ideal gas particles. ♨️
Fun Fact: Real gases behave most ideally under PLIGHT conditions: Pressure Low, Ideal gas behavior, High Temperature. H2 and He are the closest to ideal due to their small size and non-polarity.
#Kinetic Energy (KE)
Remember, temperature is directly linked to the average kinetic energy of particles. As temperature goes up, particles move faster. This is key to understanding gas behavior! 🏃
- m = mass of the molecule (kg)
- v = speed of the molecule (m/s)
- KE is measured in joules
- This formula is on the reference sheet, but understanding it is crucial!
All particles are in constant, random motion. The speed depends on temperature and other conditions. It's like a tiny, chaotic dance floor in every substance!
All gases have the same average kinetic energy at a given temperature. This is a fundamental concept that often appears in exam questions.
#The Five Assumptions of KMT
Here are the core ideas of the Kinetic Molecular Theory:
- No attractive or repulsive forces: Gas particles don't attract or repel each other.
- Negligible volume: Gas particles are tiny and far apart, so their volume is considered zero.
- Random, straight-line motion: Gas particles move randomly in straight lines until they collide.
- Elastic collisions: When particles collide, they transfer energy without any net loss.
- KE and velocity are related: KE = 1/2mv^2; all gases have the same average KE at a given temperature.
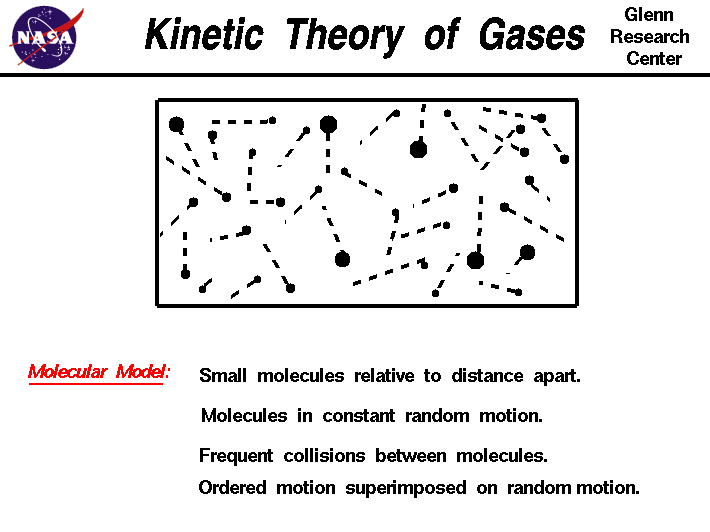
#Photo Courtesy of NASA
# Maxwell-Boltzmann Distributions
These distributions show how energy (and speed) is distributed among gas particles at different temperatures. Think of it as a speed chart for gas particles.🌡️
A common mistake is thinking that a higher peak on the distribution means more energy. It actually means that a higher number of particles have that energy.

#Image Courtesy of DeepAI
- X-axis: Speed (and energy, since KE = 1/2mv^2)
- Y-axis: Number of molecules
Notice how the cold gas has a tall, narrow peak at lower speeds, while the hot gas has a wider, flatter peak at higher speeds. This means that at higher temperatures, more molecules have higher speeds and thus more energy.
💡Remember: As temperature increases, the range of velocities becomes larger, and particles move at higher speeds. Lighter gases have the same effect. Think of it like a race: higher temperature = faster cars, lighter gas = faster cars.
Higher temperature = wider, flatter curve; lighter gas = wider, flatter curve. This is a quick way to analyze Maxwell-Boltzmann diagrams.
# AP Free-Response: 2019 #4
Let's tackle a real AP question! This is from the 2019 exam, FRQ #4. It's a great example of how KMT is applied.
Question:
A student is experimenting with CO2(g) in a rigid container. Initially, the gas is at 299 K and 0.70 atm. The student heats the gas to 425 K.
- Describe the effect of raising the temperature on the motion of the CO2(g) molecules.
- Calculate the pressure of the CO2(g) at 425 K.
- Explain, using the kinetic molecular theory, why the pressure changes as the gas is heated.
#Sample Responses
First, let's note the given information:
- Rigid container → fixed volume
- Initial Temperature (T1) = 299 K
- Initial Pressure (P1) = 0.70 atm
- Final Temperature (T2) = 425 K
##1 Breakdown
Describe the effect of raising the temperature on the motion of the CO2(g) molecules. Remember to use the phrase "average kinetic energy".
Sample Response: Increasing the temperature increases the average kinetic energy of the CO2(g) molecules, which causes them to move faster.
##2 Breakdown
Calculate the pressure of the CO2(g) at 425 K. Since the volume is constant, we can use Gay-Lussac's law: P1/T1 = P2/T2.
Sample Response: 0.99 atm
Always show the formula, substitution, and final answer with units for full credit. This is an easy way to avoid losing points.
##3 Breakdown
Explain why the pressure changes using the kinetic molecular theory.
Sample Response: Faster-moving gas particles collide more frequently and forcefully with the container walls, increasing the overall pressure.
# Final Exam Focus
Okay, you're almost there! Here's what to focus on for the exam:
-
KMT Assumptions: Know them inside and out. They are the foundation for understanding gas behavior.
-
Maxwell-Boltzmann Distributions: Understand what the axes represent and how temperature and molar mass affect the curves.
-
Gas Laws: Practice using the combined gas law and its variations (like Gay-Lussac's law).
-
Connecting Concepts: Be ready to explain gas behavior using KMT principles. AP questions often combine multiple concepts.
- Time Management: Don't spend too long on one question. If you're stuck, move on and come back later.
- Read Carefully: Pay attention to what the question is asking. Underline key words and phrases.
- Show Your Work: Always show your work, even for simple calculations. It helps with partial credit.
- Units: Always include units in your answers. It's an easy way to gain or lose points.
PLIGHT: Pressure Low, Ideal Gas, High Temperature (conditions for ideal gas behavior).
# Practice Questions
Let's test your knowledge with some practice questions!
Practice Question
Multiple Choice Questions
-
Which of the following statements best describes the behavior of an ideal gas according to the kinetic molecular theory? (A) Gas particles have significant attractive forces. (B) Gas particles have a negligible volume. (C) Gas particles move in curved paths. (D) Collisions between gas particles are inelastic.
-
A gas sample is heated from 300 K to 600 K. According to the Maxwell-Boltzmann distribution, what happens to the distribution of molecular speeds? (A) The distribution narrows and shifts to lower speeds. (B) The distribution narrows and shifts to higher speeds. (C) The distribution broadens and shifts to lower speeds. (D) The distribution broadens and shifts to higher speeds.
-
A rigid container contains a gas at a pressure of 2 atm and a temperature of 300 K. If the temperature is increased to 450 K, what is the new pressure? (A) 1.33 atm (B) 3 atm (C) 4 atm (D) 6 atm
Free Response Question
A 10.0 L rigid container contains 0.500 moles of an ideal gas at 25°C. The gas is then heated to 100°C.
(a) Calculate the initial pressure of the gas in the container.
(b) Calculate the final pressure of the gas in the container.
(c) Describe the change in the average kinetic energy of the gas molecules as the temperature is increased.
(d) Sketch a Maxwell-Boltzmann distribution curve for the gas at 25°C and 100°C on the same graph. Label the axes and curves.
Answer Key
Multiple Choice Questions
- (B)
- (D)
- (B)
Free Response Question
(a) Use the ideal gas law PV = nRT to calculate the initial pressure. Make sure to convert temperature to Kelvin (25°C = 298 K).
(1 point for correct setup, 1 point for correct answer)
(b) Use Gay-Lussac's law (P1/T1 = P2/T2) since the volume is constant. Convert temperatures to Kelvin (100°C = 373 K).
(1 point for correct setup, 1 point for correct answer)
(c) As the temperature increases, the average kinetic energy of the gas molecules increases. This is because temperature is directly proportional to the average kinetic energy of the molecules.
(1 point for mentioning that kinetic energy increases with temperature)
(d) The Maxwell-Boltzmann distribution curve should show two curves, one for 25°C and one for 100°C. The curve for 100°C should be broader and flatter, with a peak shifted to the right (higher speeds). The axes should be labeled as "Number of Molecules" (y-axis) and "Molecular Speed" (x-axis).
(1 point for correct labeling of axes, 1 point for correct shape and relative position of the curves)
You've got this! Go ace that exam! 💪
Continue your learning journey

How are we doing?
Give us your feedback and let us know how we can improve





